back to Pet Cases
3/21/2016
This was an Alcor case and perfusion was performed at our facility. The brain was then transported to Alcor for storage.
History
Canine
Male
Boston Terrier
Born: 12/3/2001
Age: 14 years, 3.5 months
Weight: 6.8 kg (15 lbs)
5 days prior to procedure, began taking 80 mg. aspirin per day.
Procedure
This was a planned event. A local veterinarian came to our facility to perform the euthanasia. Temperatures listed below are from the nasopharyngeal thermocouple.
| Time | Time PP | |
| 10:10 | Veterinarian started an IV in the right foreleg | |
| 10:12 | Veterinarian administered 10,000 units heparin sodium (10 mL 1,000 USP Units/mL) through IV line. | |
| 10:15 | Veterinarian administered 2.5 mL SomnaSol | |
| 10:16 | Veterinarian administered 0.2 mL TTDex | |
| 10:17 | 0:00 | Veterinarian pronounced death. |
| 10:17 | 0:00 | Veterinarian administered 750 mg (3.75 mL) Sodium Citrate 200 mg/mL into IV line. |
| 10:18 | 0:01 | Placed in ice bath on right side, and began chest compressions. |
| 10:29 | 0:12 | Placed a thermocouple into the right nostril, 4 cm to nasopharynx. Secured with tape. |
| 10:32 | 0:15 | Nose was lifted out of ice water, and nasal temp was disregarded as inaccurate until about 0:32 PP |
| 10:38 | 0:21 | Placed a rectal occlusion device with thermocouple on the side. |
| 10:48 | 0:31 | Removed some water from ice bath in preparation for surgery. |
| 10:49 | 0:32 | Surgery started. Aorta was not easily accessible, so brachiocephalic artery was isolated. |
| 11:19 | 1:02 | Cannula inserted into brachiocephalic artery and 3 cm up into the left common carotid artery. |
| 11:20 | 1:03 | Washout, B1 started. Incision made in right atrium for effluent. |
| 11:42 | 1:25 | Heat exchanger iced up. Paused for 5 minutes to melt. Resumed. |
| 11:51 | 1:34 | Cryoprotective perfusion began. Flow into artery remained good throughout procedure. |
Perfusion Circuit
The open circuit consisted of the following components:
Bags of perfusate, 1 L each
Y-tubing for attaching 2 bags at the same time. Only one bag is flowing. This is how bags are switched.
Roller pump with 3/16 ID shoe, LS15
Heat exchanger, LN2 chiller
Inline refractometer
Analog manometer connected via isolater
Digital pressure transducer
40 micron filter
Temperature probes. One nearer to the chiller and one nearer to the patient.
Arterial cannula, right angle
Perfusion
It was a stepped nM22 perfusion with 11 steps. The times listed below are for when each bag was started.
| Time | Time PP |
Bag Label | Brix | kg used | Notes |
| 11:20 | 1:03 | B1 | 9.6 | 1.03 | |
| B1 (2nd) | 10.2 | 0.65 | |||
| 11:51 | 1:34 | 2 | 0.51 | ||
| 11:59 | 1:42 | 3 | 0.49 |
||
| 12:07 | 1:50 | 4 | 12.9 | 0.52 | |
| 12:16 | 1:59 | 5 | 18.4 | 0.52 | |
| 12:28 | 2:11 | 6 | 25.2 | 0.53 | |
| 12:43 | 2:26 | 7 | 27.3 |
0.54 | |
| 12:58 | 2:41 | 8 | 50.7 | 0.55 | |
| 13:15 | 2:58 | 9 | 49.8 | 0.53 | |
| 13:32 | 3:15 | 10 | 50.6 | 0.55 | |
| 13:49 | 3:32 | 11 | 50.8 | 0.46 |
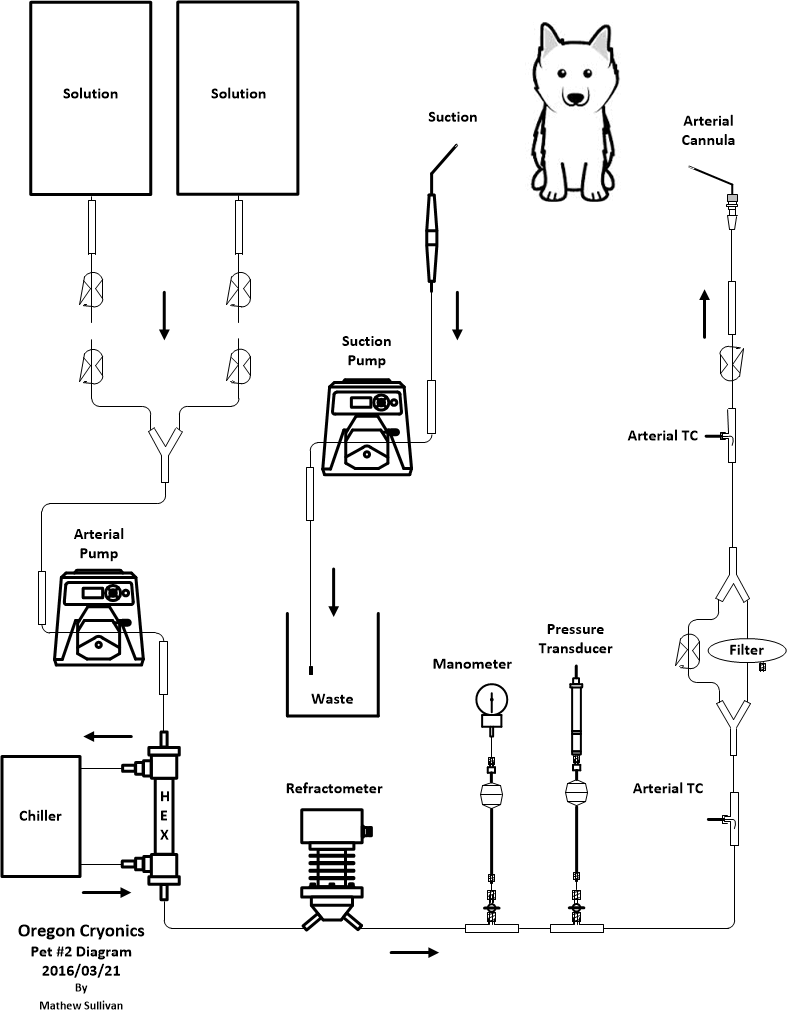
Temperature
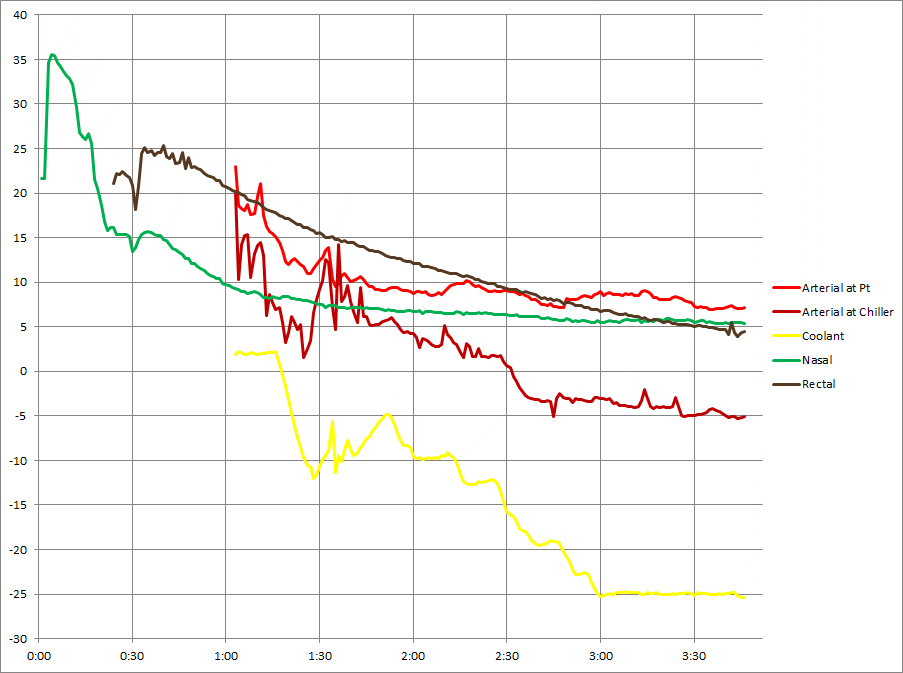
Pressure
These are line pressures, not patient pressures. The pressures prior to about 2:00 PP may be off by up to 5 mmHg due to pressure calibration issues.
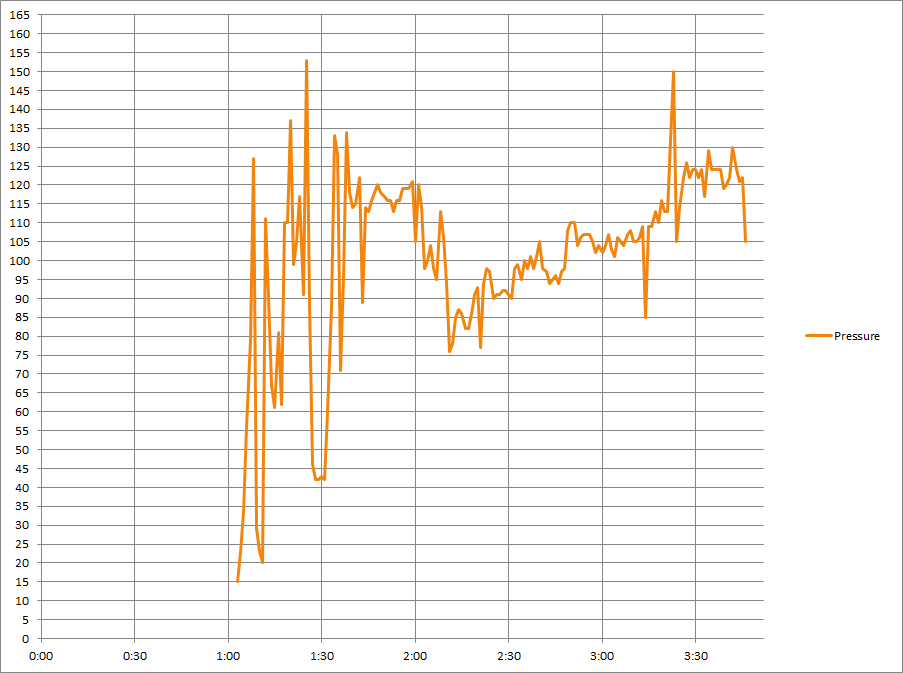
Refractive Index
Dissection
At 14:10 (3:53 PP), dissection began for access to the brain. The procedure took place inside of a temperature controlled acrylic enclosure.
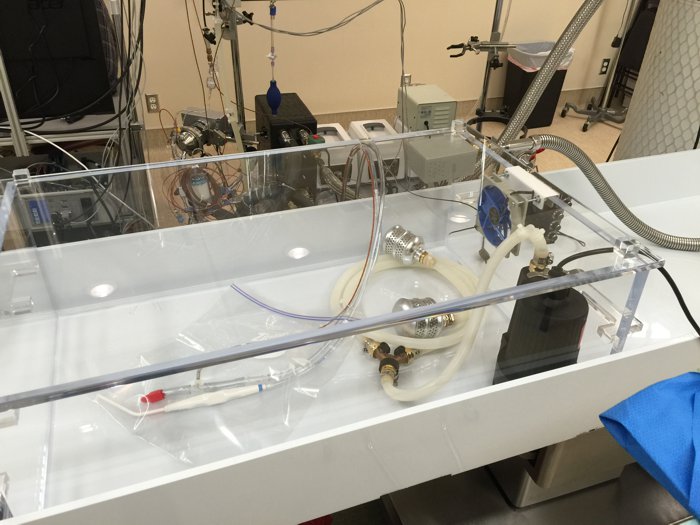
The enclosure was used as an icebath for stabilization, washout, and cryoprotection. After perfusion was complete, Cold nitrogen gas was turned on and injected by computer control a few times per minute in order to maintain the temperature inside the enclosure at zero degrees Celcius. Access was through the top of the enclosure, and visibility was quite good in spite of brief periods of fogging.
Cephalic isolation was performed. Skin and muscle were removed from the posterior and superior of the skull. Rongeurs and bone saw were used to free as much of the skull in that area as possible. The bone saw was constantly irrigated with cold M22 to avoid warming. The dura mater was removed. No noticeable brain shrinkage was observed. Biopsies were taken at 14:40 (4:23 PP) for further analysis. Cranial nerves were transected, and the brain was removed at 14:53 (4:36 PP).
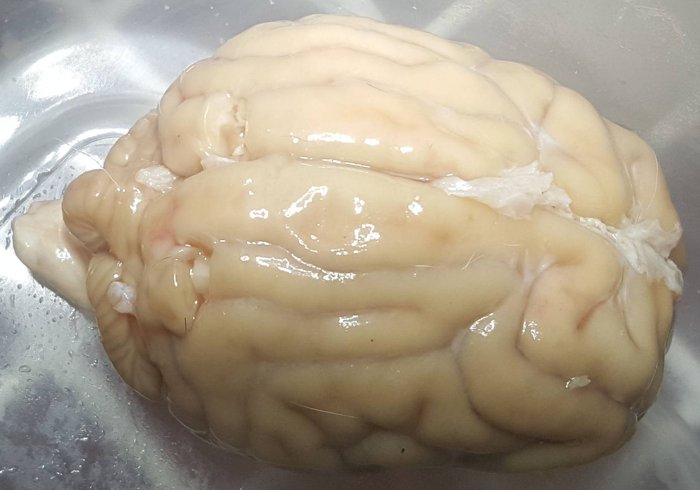
Biopsy locations are visible in the photo at the left.
Concentrated M22 was drizzled on the surface, and a thermocouple was placed between the hemispheres. The brain was placed in a closed plastic container, on dry ice, in a precooled LR-40 dewar. Cooling continued on a ramp for 35 hours to -196
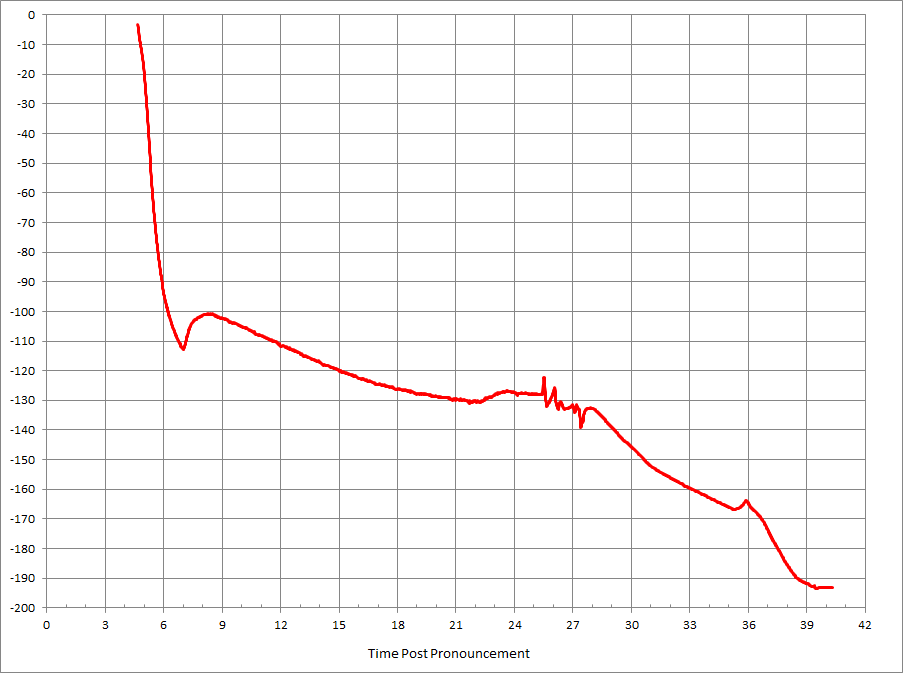
It was then transferred to LN2. It was shipped overnight to Alcor a week later in a dry shipper, and the temperature logger showed that the temperature stayed at -196 for the entire trip.
A followup dissection of the major arteries was performed a few days later. The aorta had two branches: brachiocephalic and left subclavian. 2 cm from the aorta, the left common carotid branched off from the brachiocephalic, and this was the artery that was used by us for perfusion. 2 cm further, the brachiocephalic forked to become the right subclavian and the right common carotid.
Discussion
Using stepped bags did not seem very efficient. It required significant time to manage and switch the bags.
Temperature rise between the chiller and the patient was a real problem. It was mainly caused by the large metal refractometer, the slow flow, and the inability to use the cooling enclosure due to having to hold the cannula throughout the case.
Manual arterial pump control was used for a number of reasons. The pressure graph would have looked smoother with automation.
Quality
Ischemic Time Preperfusion
Since CPS was started immediately, we assume there was adequate cerebral perfusion for the first 10 minutes of CPS, and that 10 minute span is excluded from the contribution towards the score. Because of ice water on the nasal thermocouple, the temperature was interpolated for the first 30 minutes.
Score: 15 minutes
Ischemic Time Total
Calculated until 4:36 PP using nasal temp
Score: 33 minutes
