If a brain is simply frozen without any preparation, then ice crystals would form. As the ice expands, it would crush the delicate structures we are trying to preserve. So, prior to cooling to liquid nitrogen temperature, we go to a lot of effort to ensure adequate concentration of cryoprotective agent (CPA). It's expensive and complex.
At some point, most people will ask the question of whether we might skip the CPA anyway. The reasoning goes that the expansion of the ice follows a predictable trajectory, so the supercomputers of the future might be able to infer the original positions of the molecules. And since it's faster, maybe it would capture the molecules earlier and also prevent various other iatrogenic damage.
I'm skeptical of this approach. I think the picture below sums up why.
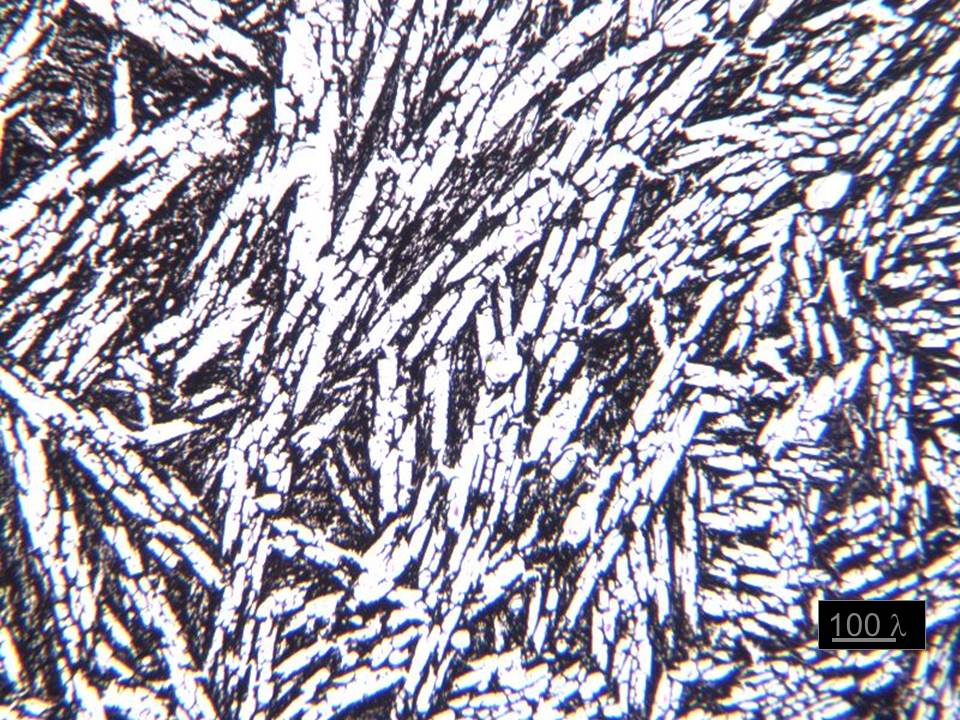
This is a frozen rabbit brain under a light microscope. The white areas are ice, and the dark areas are the crushed and flattened brain tissue. Can the original structure be inferred? I don't think so. The crushing action of ice is like a mortar and pestle. It doesn't just make it flat with compression forces, but it also "smears" the material with shearing forces between 25,000 and 114,000 psi. Any physical process that stirs or smears molecules causes a kind of damage which would not allow future inferrence of the original structure. You just can't unmix something. There's not enough information.
Based on this photo, we work very hard to ensure adequate CPA concentration everywhere before final cooling. In the cases where we need more time to let the CPA diffuse in, then we depend on aldehydes to temporarily hold the structure as best as they are able. When we can't perfuse, it can take many weeks for the CPA to diffuse in. That seems so much better than smashing everything without strong evidence that it's reversible.
